Hand hygiene and transmission of Pseudomonas aeruginosa on hands in a hospital environment
Hand hygiene and transmission of Pseudomonas aeruginosa on hands in a hospital environment
Susan Jones
Health Protection Agency – Microbiology Services, Food Water and Environmental Microbiology Laboratory, Southampton, Hampshire, SO16 6YD, UK. Email: [email protected]
Accepted for publication: 11 February 2011
Key words: Hand hygiene, Pseudomonas aeruginosa contamination, infection control
Abstract
Colonisation and infection with Pseudomonas aeruginosa from the water supply constitutes a risk inneonatal hospital units. This short research notedemonstrates that the use of correct hand hygiene minimizesthe risk of contamination of hands with P. aeruginosa even when heavily contaminated wash water isused.
Introduction
Linking hand hygiene practices and infection or colonisation with Pseudomonas aeruginosa in neonatal units is well-established ( Crivaro et al, 2009 ; Lam et al, 2004 ). A very recent letter from the Department of Health has drawn attention to contamination of hospital water supplies with Pseudomonas , and the risk imposed on augmented care wards ( Department of Health, 2010 ). Environmental surveillance at a large teaching hospital was instigated following colonisation of neonates in a neonatal unit. Test results indicated persistent point-colonisation of hospital water supplies with P. aeruginosa. The aim of this study was to investigate whether good hand hygiene using standard procedure (NHS Trust Hand Hygiene Policy, based on Part II Consensus Recommendations, WHO Guidelines, 2009) is sufficient to remove contamination with P. aeruginosa , even where heavily contaminated water is used.
Method
Tap water heavily colonised with P. aeruginosa in another part of the hospital was used to investigate hand hygiene practices (HHP). Personnel in this study comprised two groups: infection control staff for whom this training is entrenched practice, and laboratory personnel. Although all staff working in the clinical environment, including laboratory staff, receive handwashing training (mandatory trust policy) that takes 40–60 seconds if carried out correctly, departure from standard practice may occur, particularly in a busy, and possibly overstretched, hospital. Laboratory staff were either instructed to wash their hands ‘as they would normally do’ (which may equate to usual practice for non-specialist infection control staff) or according to HHP training, following the six-point hand hygiene fl ow diagram ( trust policy, based on WHO, 2009 ) given as a reminder. Unless stated otherwise, paper towels were used for hand drying. No specific instruction was issued to infection control staff, unless there was a departure from the standard practice (i.e. gel use on wet hands).
Water samples before, during and after each trial, and environmental swabbing of hand gel and soap dispensers were taken concomitantly to ensure consistent colonisation of the water supply and to eliminate the possibility of other contamination routes. P. aeruginosa was detected using SpongeSicle ™ swabs: swab samples were homogenised with peptone saline diluent to extract bacteria, the diluent was fi ltered and the fi lter membrane placed on selective agar medium (PCN agar Oxoid™; method based on BS ISO 12780 ).
Only areas of hands likely to come into contact with other surfaces were swabbed, i.e. palms and fingers, particularly the finger tips. Water samples were processed according to BS ISO 12780 . Comparative statistics were not applied to data due to the low and variable sample numbers in each trial.
Results
The tap water yielded initial mean contamination levels of 2680 P. aeruginosa per ml (n = 5, range 420–5000), and samples were taken for P. aeruginosa determination immediately before, during and after each separate handwash trial. Contamination levels during and immediately after each trial were reduced to an average 500 P.aeruginosa per ml (n = 12), but were never lower than 100 colony forming units (cfu) per ml. P. aeruginosa was not detected on hands prior to washing in contaminated water (n = 24) nor was it present on the gel dispenser, but was occasionally found on the soap dispenser handle. This emphasised that the contamination route was via the water and not the dispensers, since the soap dispenser was invariably operated using pre-wetted (and contaminated) hands whereas the gel dispenser was used after hands had been dried with paper towels (with exception: see below). P. aeruginosa did not appear to be persistent on dried hands since this organism was never recovered from hands at the beginning of each handwashing trial even though several sinks that laboratory staff routinely used were known to be contaminated.
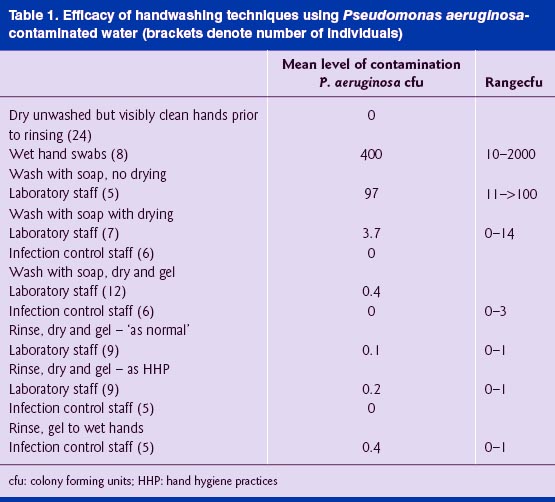
Variable but often high levels of P. aeruginosa remained on hands after rinsing (but before drying) in contaminated water, irrespective of whether soap was used. Where no particular instruction was given, comparison of hand washing with soap and drying showed low level (<10 cfu per hand swab) carryover on 43% (three out of seven) laboratory staff hands, compared with no survival on infection control staff hands ( Table 1 ). The question was posed as to whether there was any benefit in using alcohol gel as well as HHP when contaminated rinse water was used: carryover on laboratory staff hands using the combined approach was reduced to 16 % (<4 cfu per hand swab; 2 out of 12 people), compared with no survival on infection control staff hands. This suggests that a combined approach may have utility where best practice is not always heeded.
Alcohol hand gels may be used in place of soap and water only if hands are visibly clean (NHS Trust Hand Hygiene Policy; WHO Guidelines 2009). Comparisons were made between contaminated but dry hands of laboratory staff (rinse, dry and gel; Table 1 ) using ‘normal’ procedures and according to HHP. Low level carryover was found on one out of nine laboratory staff hands when ‘normal’ procedures was applied; when staff were reminded of correct HHP, the carriage rate increased slightly to two out of nine. In comparison, no carryover of P. aeruginosa was found on infection control staff hands ( n = 5) following use of gel only.
Conclusions
This study supports the long-established opinion that P. aeruginosa is uncommon on dry healthy hands ( Pillsbury et al, 1950 ) even where continued exposure is likely to have occurred via contaminated handwashing facilities. Although sample numbers were too low to apply statistical analyses, washing and drying hands according to standard hospital policy is effective, irrespective of whether the water used is heavily contaminated. Infection control staff consistently demonstrated that when the six-point hand procedure is used, carryover is avoided. In contrast, it was observed that laboratory staff frequently did not perform the standard procedure, which was evidenced in the higher carryover of P. aeruginosa in this group . Correct HHP in the absence of effective drying still ensures a very low level of survival of P.aeruginosa, even when gel is applied to wet and contaminated hands, as evidenced by low survival on wet hands of infection control staff.
As discussed by Weaving (2007) , this study underlines the importance of instilling routine embedded behaviour for hospital personnel: although laboratory staff were instructed in HHP, clearly it is not being applied as stringently as it could be and is not part of instilled behaviour. Despite the small numbers of participants, the study showed that hand gel is slightly more effective at reducing Pseudomonas than soap and water, findings alluded to elsewhere (Weaving, 2007).
However, the most effective way of reducing risk of infection is still the removal of the infective reservoir in the first place; modification to HHP should remain short-term measures only.
Acknowledgements
I am grateful for Health Protection Agency laboratory staff and NHS infection control staff for their advice and involvement.
Confl ict of interests
None declared.
References
BS EN 12780: 2002 (2002) Water quality. Detection and enumeration of Pseudomonas aeruginosa by membrane fi ltration. British Standards Institution (BSI).
Crivaro V, Di Popolo A, Caprio A, Lambiase A, Di Resta M , Borriello T, Scarcello A , Triassi M, Zarilli R. ( 2009 ) Pseudomonas aeruginosa in a neonatal intensive care unit: molecular epidemiology and infection control measures. BMC Infectious Diseases 9: 70 .
Department of Health (2010) Water sources and potential for infection from taps and sinks. https://publication.spaceforhealth.nhs.uk. Lam B, Lee J, Lau Y. ( 2004 ) Hand hygiene practices in a neonatal intensive care unit: a multimodal intervention and impact on nosocomial infection . Pediatrics 114(14 ): 565 – 71 .
Pillsbury D, Rebell G, De Saint Phalle M, Ginsberg D. ( 1950 ) Factors affecting the rapid disappearance of bacteria placed on the normal skin. J ournal of Investigative Dermatology 14 : 247 –6 4.
Weaving P. ( 2007 ) Editorial: Hand hygiene: it’s still important. British Journal of Infection Control 8 (5): 4 – 5 .
World Health Organization. World Alliance for Patient Safety ( 2009 ) Guidelines on hand hygiene in health care. WHO : Geneva.



